



- Stock: In Stock
- Model: 180012
0% Customers recommend this product
-
5 Awesome0%
-
4 Great0%
-
3 Average0%
-
2 Bad0%
-
1 Poor0%
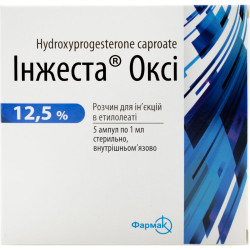
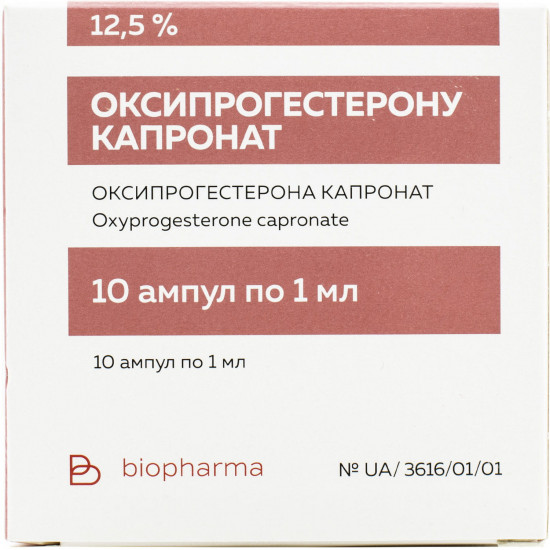
Reviews Over Pregnenoldione kapronat solution for infection. masl. 12.5% of amp. 1 ml No. 10
- (0)
Total Reviews (0)
click here write review to add review for this product.
Report this review.
Description
Translation of the instruction Mose
KAPRONAT PREGNENOLDIONE Solutio oleosa for injections of 12.5%Instruction
On medical use of medicine
Pregnenoldione kapronat
(oxyprogesterone capronate)
Ingredients:
Active ingredient: 1 ml of solution contains hydroxyprogesterone kapronat (in terms of dry matter) 125 mg;
excipients: benzyl benzoate, the olive oil refined.
Dosage form.
Solution for injections oil.
Main physical and chemical properties: transparent oily liquid from light yellow till chartreuse color.
Pharmacotherapeutic group.
Hormones of gonads and medicaments used at pathology of the sexual sphere. progesterona. code atkh g03d a03.
Pharmacological properties.
Pharmacodynamics.
Pregnenoldione kapronat is a synthetic analog of hormone of a yellow body (progesterone). In biological properties it is similar to progesterone: causes transformation of a mucous membrane of a uterus from a phase of the proliferation caused by follicular hormone in a secretory phase, and after fertilization promotes its transition to a state necessary for oospore development; reduces excitability and contractility of muscles of a uterus and uterine tubes, stimulates development of trailer elements of a mammary gland. In comparison with progesterone more racks in an organism, work more slowly and render the prolonged effect. After a single intramuscular injection from 7 to 14 days maintain the action.
Pharmacokinetics.
byAfter an intramuscular injection it is slowly absorbed from the injection site. Therapeutic concentration to be kept for 7–14 days.
Clinical characteristics.
Indication.
Patolog_chn_ of a protsesa, zumovlen_ nedostatn_styu zhovty t_l: zagroza vikidnya; pervinna that vtorinna amenorrhea; pol_menoreya; disfunkts_onaln_ matkov_ krovotech і; g_perplastichn_ protses in endometr і ї, endometr_oz.
Contraindication.
Hypersensitivity to medicine components. the second half of pregnancy, an extrauterine pregnancy or the stood pregnancy, in the anamnesis, vaginal bleedings of unspecified genesis, a state after abortion, abnormal liver functions, a liver disease (hepatitis, cirrhosis), cholestatic jaundice during pregnancy or in the anamnesis, a benign hyperbilirubinemia, a liver failure, a renal failure, a porphyria, nervous breakdowns with the depression phenomena, tachycardia, malignant tumors of mammary glands and genitals, an active venous or arterial thrombembolia, heavy thrombophlebitis or such states in the anamnesis.
Interaction with other medicines other types of interactions.
Pregnenoldione kapronat is weakened by effect of medicines which stimulate myometrium reduction (oxytocin, Pituitrinum), anabolic steroids (retabolil, Nerobolum), gonadotropic hormones of a hypophysis. in interaction with oxytocin the lactogenic effect decreases. strengthens effect of diuretics, hypotensive medicines, immunodepressants, a bromkriptin and system coagulants. reduces efficiency of anticoagulants. changes effects of gipoglikemiziruyushchy means. the gestagenny activity is reduced by inductors of microsomal oxidation (carbamazepine, griseofulvin, barbiturates, hydantoins, rifampicin). combined use
β-adrenomimetik and hydroxyprogesterone of the kapronat for prevention of premature births promotes reduction of side effects of β-adrenomimetik.
Hydroksiprogesteron'skapronat cyclosporine metabolism oppresses that leads to increase in concentration of cyclosporine in plasma of blood and risk of emergence of toxic effects.
Feature of application.
With care should use medicament to patients with arterial hypertension, cardiovascular diseases, diabetes, bronchial asthma, epilepsy, migraine, a depression.
to applyWith care to patients with other diseases which promote a liquid delay of the patient with mental violations in the anamnesis, medicine needs to be cancelled at emergence of the first symptoms of a depression.
should not use medicament to patients with rare hereditary diseases, such as intolerance of a galactose, insufficiency of lactase, glucose galactose malabsorption.
At patients with diabetes needs to control carefully glucose indicators in blood. It is not necessary to use medicament at bleedings from a genital tract which reason is not established, and to patients in whose anamnesis diseases of peripheral arteries were noted. At use of medicine it is necessary to be attentive to various symptoms and symptoms of a thrombembolia, and in case of their emergence the therapy by medicine needs to be stopped.
toduring treatment recommends carrying out regular surveys which frequency and volume are defined individually.
in the presence of any progestogenzavisimy tumor, for example, of a meningioma in the past and/or its progress during pregnancy or the previous hormonal therapy patients have to be under careful observation of the doctor.
At prolonged use of high doses the termination of periods is possible.
Use during pregnancy or feeding by a breast.
to use Drug only in і a pregnancy trimester at threat of an abortion. the risk of congenital anomalies, including sexual anomalies at children of both sexes, is connected with effect of exogenous progesterone during pregnancy, completely is not established. progesterone gets into breast milk therefore it is not necessary to use medicament during feeding by a breast.
Ability to influence speed of response at control of motor transport or work with other mechanisms.
Drug can cause disorders of vision and increased fatigue. during treatment it is necessary to abstain from occupations potentially dangerous types of activity requiring special attention and speed of psychomotor reactions.
Route of administration and doses.
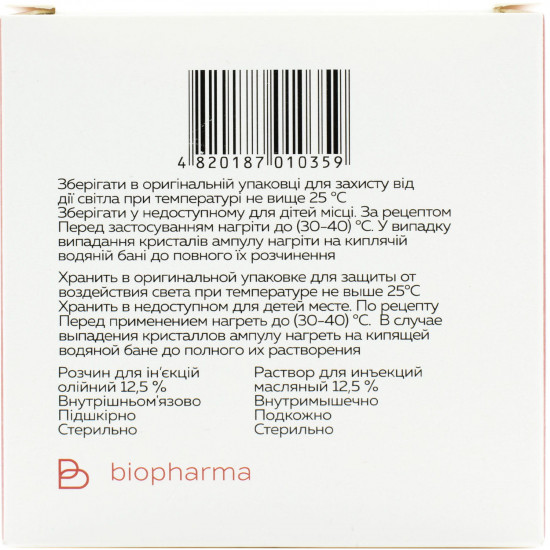
an ampoule with medicine slightly to warm upBefore use on the water bath (till 30-40 ºс). in case of loss of crystals to warm up an ampoule on the boiling water bath before their full dissolution. to enter intramusculary and subcutaneously.
in І a trimester of pregnancy to enterAt the menacing or begun abortion on 125–250 mg (1–2 ml of 12.5% of solution) once a week.
At primary or secondary amenorrhea — directly after phase-out of estrogenic medicines to enter 250 mg once or to divide into two receptions.
for the purpose of normalization of a menstrual cycle (at a polymenorrhea, dysfunctional uterine bleedings) to administer the medicament in a dose 65–125 mg (0.5-1 ml of 2.5% of solution) on
20-22nd day of a cycle.
byto Women with an endometrium hyperplasia (in the absence of hormonal active tumors of ovaries) up to 45 years in the I phase of a menstrual cycle to appoint estrogen (ethinylestradiol of 0.05 mg a day from the 5th to the 25th day of a cycle) and Pregnenoldione kapronat on 1 ml of 12.5% of solution to the 5th, 12th and 19th day of a cycle during 4–5 cycles once a week. To women from 45 years to enter only Pregnenoldione kapronat on 2 ml 12.5% of solution during 6–8 menstrual cycles once a week.
Children.
to children is not presentExperience of use of medicine therefore it cannot be applied in pediatric practice.
Overdose.
At application of the raised medicine doses more often the side effects described in appropriate section arise. at emergence of gestagenzavisimy side effects the treatment needs to be stopped, and after their disappearance — to continue
in smaller doses. In case of need to carry out symptomatic treatment.
Side reactions.
from a cardiovascular system: increase in arterial blood pressure, tachycardia, short wind, blood circulation violation, venous a thrombembolia is possible.
from a metabolism: hypostases, a liquid delay, an albuminuria, bad tolerance of glucose, change of a lipidic profile of plasma are possible.
from digestive system: change of appetite, an abdominal distension, an abdominal pain, a lock, diarrhea, an abnormal liver function and change of functional trials of a liver, cholestatic jaundice, is rare (at prolonged use) — nausea, vomiting.
from the central nervous system (CNS): seldom (at prolonged use) — a headache, dizziness, a depression, insomnia, drowsiness, weakness, increased fatigue, paresthesias.
from an endocrine system: seldom (at prolonged use) — increase in body weight, pain and tension in mammary glands, change of vaginal discharge, irregular uterine bleedings, an amenorrhea, an oligomenorrhea, violation of a menstrual cycle, a premenstrual syndrome, decrease in a libido, a hirsutism.
from genitals: spasms of a uterus, violation from external genitals such as burning, dryness, genital itch, vaginal discharges, vaginal mycosis.
from the immune system: reaction of hypersensitivity, anaphylactic reactions, Quincke's disease, small tortoiseshell.
from skin and hypodermic fabric: the alopecia, an acne, a yellow nevus pigmentosus on a face (hloazm), are possible allergic reactions on skin (rash, an itch), a multiformny erythema.
from organs of sight: disorder of vision, retina thrombosis, inflammation of optic nerves.
General violations and changes in the injection site: fever; changes in the injection site, including pain and a swelling.
Influence on a fruit: the excess amount of progesterone can cause virilescence of a female fruit (up to uncertainty of sex).
Expiration date.
5 years.
Storage conditions.
to Storein original packing for protection against influence of light at a temperature not above 25 °C. to store out of children's reach.
Incompatibility.
Drug should not be mixed with other medicines in one capacity.
Packing.
On 1 ml in an ampoule; on 5 ampoules in the blister; on 2 blisters in a pack from cardboard.
Category of a holiday.
According to the prescription.
Producer.
LLC fz «biofarma, Ukraine.
Location of the producer and its address of the place of implementation of activity.
Ukraine, 09100, Kiev Region, white church, st. Kiev, 37.
Specifications
| Characteristics | |
| Active ingredients | Hydroksiprogesterona kapronat |
| Amount of active ingredient | 125 mg/ml |
| Applicant | Biopharma |
| Code of automatic telephone exchange | G03DA03 Hydroksiprogesteron |
| Interaction with food | It doesn't matter |
| Light sensitivity | Sensitive |
| Market status | Generic-generic |
| Origin | Chemical |
| Prescription status | According to the prescription |
| Primary packing | ampoule |
| Producer | BIOFARM OF THE FEDERAL LAW OF LTD COMPANY |
| Quantity in packing | 10 ampoules on 1 ml |
| Release form | solution for injections |
| Route of administration | Intramuscular |
| Sign | Domestic |
| Storage temperature | from 15 °C to 25 °C |
| Trade name | Pregnenoldione kapronat |