
- Stock: In Stock
- Model: 185248
0% Customers recommend this product
-
5 Awesome0%
-
4 Great0%
-
3 Average0%
-
2 Bad0%
-
1 Poor0%
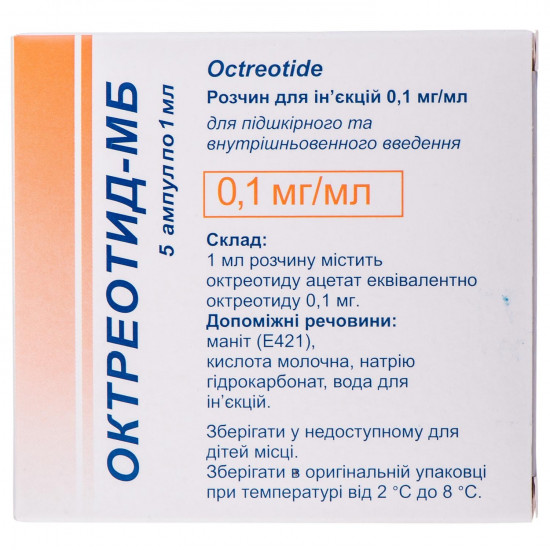
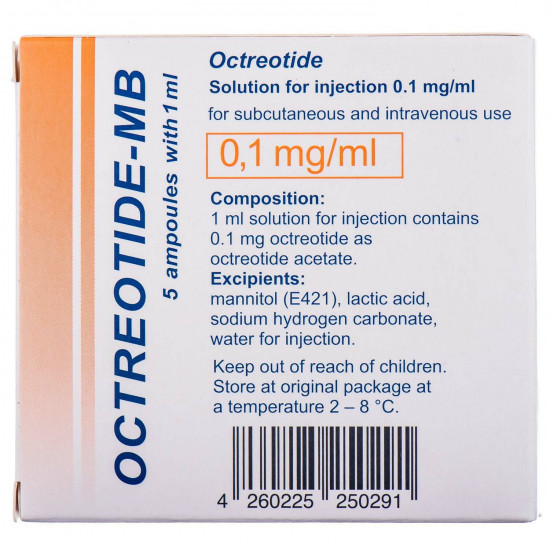
Reviews Over Oktreotid-MB solution for infection. 0.1mg/ml amp. 1 ml No. 5
- (0)
Total Reviews (0)
click here write review to add review for this product.
Report this review.
Description
Solution is applied to injections of "Oktreotid-MB" at indications which are provided below.
Acromegalia, for control of the main manifestations of a disease and decrease in level of the growth hormone (GH) and an insulinopodobny growth factor 1 (IFR-1) in blood plasma in case there is no sufficient effect of surgical treatment and radiation therapy. Drug is shown also for treatment of the patients with an acromegalia who refused operation or having contraindications to it and also for short-term treatment in intervals between courses of radiation therapy until its effect completely does not develop.
Relief of symptoms connected with endocrine tumors of a digestive tract and pancreas:
- carcinoid tumors with existence of a carcinoid syndrome; VIPoma's
- (the tumors which are characterized by hyperproduction of vasoactive intestinal peptide);
- glucagonoma;
- gastrinoma / Zollingera-Ellison's syndrome, usually in combination with antagonists of H2 receptors or inhibitors of the proton pump;
- to an insulinoma (for control of a hypoglycemia in the preoperative period and also for maintenance therapy);
- a somatoliberinoma (the tumors which are characterized by hyperproduction of a rileasing-factor of growth hormone).
"Oktreotid-MB" is not antineoplastic medicament therefore its use cannot promote treatment of this category of patients.
Prevention of complications after pancreas surgeries.
Termination of bleedings and prevention of a recurrence of bleedings from varicose expanded veins of a gullet at patients with cirrhosis (in a combination with specific treatment, for example, with endoscopic sclerosing therapy).
Structure
Active ingredient - octreotide (1 ml of solution supports an oktreotid acetate it is equivalent to an oktreotid of 0.1 mg).
Excipients: a mannitol (E 421), lactic acid, Natrii hydrocarbonas, water for injections.
Contraindication
Known hypersensitivity to an oktreotid or other components of drug.
Route of administration
In an acromegalia originally the medicament is administered on 0.05-0.1 mg subcutaneously by everyone 8 or 12 hours. Further selection of a dose has to be based on monthly definitions of concentration of the growth hormone (GH) and an insulinopodobny growth factor 1 (IFR-1) in blood, the analysis of clinical symptoms and tolerance of medicament (target concentration of hormones in blood make: GR - less than 2.5 ng/ml, IFR-pervykh norm limits). For most of patients the optimum daily dose makes 0.3 mg. The maximum daily dose makes 1.5 mg a day which should not be exceeded. At the patients receiving a stable dose of an oktreotid it is necessary to define concentration of GR and IFR-1 each 6 months. If within 3 months of the Oktreotid-MB medicament treatment the sufficient decrease in the GR level and improvement of a clinical picture of a disease is not noted, therapy should be stopped.
In endocrine tumors of a GIT and pancreas the medicament is administered subcutaneously in an initial dose of 0.05 mg by 1-2 times a day. Further, depending on the reached clinical effect and also influence on levels of the hormones produced by a tumor (in case of carcinoid tumors - influences on discharge of 5-hydroxyindolacetic acid with urine), and shipping, it is possible to increase a dose gradually to 0.1-0 2 mg 3 times a day. In exceptional cases higher doses can be required. Maintenance doses of medicament should be selected individually. If within one week of the Oktreotid-MB medicament treatment in the most tolerable dose the improvement does not occur, therapy should be stopped.
For prevention of complications after pancreas surgeries the medicament is administered subcutaneously on 0.1 mg by 3 times a day within 7 consecutive days, since day of operation (at least in 1 hour prior to laparotomy).
In bleeding from varicose expanded veins of a gullet administer the medicament in a dose of 25 mkg/hour by continuous infusion within 5 days. "Oktreotid-MB" it is possible to part 0.9% with chloride sodium solution. At patients with cirrhosis and bleedings from varicose expanded veins of a stomach and a gullet the good susceptibility to an oktreotid when receiving a stable dose of medicament to 50 mkg/h in the form of continuous infusion within 5 days was noted.
Feature of use
Pregnant
as a precautionary measure it is desirable for p to avoid use of an oktreotid during pregnancy.
Is forbidden to be nursed during treatment reparaty "Oktreotid-MB".
Does not know towhether affects oktreotid fertility of the person.
Children toto Children use of this medicament is contraindicated to
due to the lack of clinical experience. Drivers
"Oktreotid-MB" has no
or has insignificant influence on ability to run vehicles and other mechanisms. Patients should recommend to show care when driving or during the work with other mechanisms if they feel giddy, an asthenia / increased fatigue or a headache during treatment oktreotidy. to Overdose
Knows to
limited number of accidental overdose oktreotidy adults and children. At adults of a dose were in range of 2400-6000 mkg/day at introduction by continuous infusion (100-250 mkg/hour) or subcutaneously (1500 mkg 3 times a day). It was reported about such by-effects: arrhythmia, arterial hypotension, cardiac arrest, brain hypoxia, pancreatitis, liver steatosis, diarrhea, weakness, drowsiness, loss of body weight, hepatomegalia and milk acidosis.
At children of a dose of an oktreotid were made by 50-3000 mkg/days and were entered by continuous infusion (2.1-500 mkg/hour) or subcutaneously (50-100 mkg). The easy hyperglycemia was the only undesirable phenomenon.
byAt the patients with cancer receiving doses of an oktreotid of 3000-30000 mkg/days in the form of the divided doses subcutaneously the unexpected undesirable phenomena it was not observed.
Treatment: symptomatic therapy.
Side effects
at treatment oktreotidy belong disturbances from a digestive tract, nervous system, a liver and a gall bladder, metabolism and a trophicity To the most frequent side reactions. Side reactions about most of which often reported during conduct of clinical trials of an oktreotid were: diarrhea, abdominal pain, nausea, meteorism, headache, cholelithiasis, hyperglycemia and constipation. Belonged to other side reactions about which often reported: dizziness, local pain, bilious concrements, dysfunction of a thyroid gland (for example the lowered level of tireostimuliruyushchy hormone, reduced level of the general T4 and reduced level of free T4), a liquid chair, the tolerance to glucose, vomiting, an asthenia and a hypoglycemia is broken.
Storage conditionsto Store
in original packing at a temperature from 2 °C to 8 °C, out of children's reach.
Expiration date - 5 years.
in the course of use of an ampoule can be held at the room temperature up to 2 weeks.
Specifications
| Characteristics | |
| Active ingredients | Oktreotid |
| Amount of active ingredient | 0.1 mg/ml |
| Applicant | M.Biotech |
| Code of automatic telephone exchange | H01CB02 Oktreotid |
| Interaction with food | It doesn't matter |
| Light sensitivity | Not sensitive |
| Market status | Generic-generic |
| Origin | Chemical |
| Prescription status | According to the prescription |
| Primary packing | ampoule |
| Producer | BENDALIS GMBH |
| Quantity in packing | 5 ampoules on 1 ml |
| Release form | solution for injections |
| Route of administration | Intravenous |
| Sign | Import |
| Storage temperature | from 2 °C to 8 °C |
| Trade name | Oktreotid |